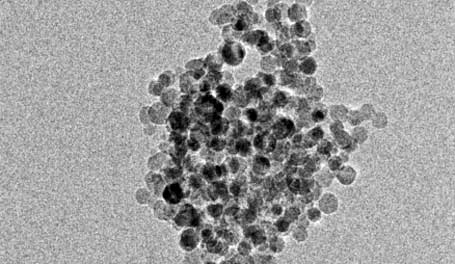
University of Buffalo researchers have discovered that hydrogen on demand may be accomplished simply by adding water to nano-particle sized silica. The researchers believe they can produce more hydrogen using this method that other common methods such as using aluminum.
According to the University of Buffalo, “In a series of experiments, the scientists created spherical silicon particles about 10 nanometers in diameter. When combined with water, these particles reacted to form silicic acid (a nontoxic byproduct) and hydrogen – a potential source of energy for fuel cells.
“The reaction didn’t require any light, heat or electricity, and also created hydrogen about 150 times faster than similar reactions using silicon particles 100 nanometers wide, and 1,000 times faster than bulk silicon, according to the study.”
The researchers believe that this could be a solution for portable fuel cell applications such as the Military on field missions or campers who need power off the grid. But, if this process works as advertised, then I say why look towards scaling up this process? Silica or silicon dioxide as it’s also know is one of the most abundant chemical compounds on Earth (as in sand or quartz).
Besides I dream of one day driving my hydrogen car around the beach cities of California where surf and sand constantly meet and I think it would be very poetic if this is how the H2 refueling stations also created their fuel.
Comments are closed here.