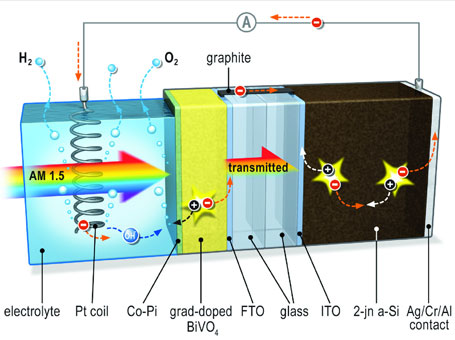
In Germany, the HZB Institute for Solar Fuels and TU Delft University of Technology have created a solar device that can split water into hydrogen cheaply and efficiently. On the solar cell, the anode is made from metal oxide bismuth vanadate (BiVO4) plus a small amount of tungsten atoms with a dash of wolfram atoms thrown in for good measure.
According to HZB, “Thus the experts were able to develop a rather elegant and simple system for using sunlight to split water into hydrogen and oxygen. This process, called artificial photosynthesis, allows solar energy to be stored in the form of hydrogen. The hydrogen can then be used as a fuel either directly or in the form of methane, or it can generate electricity in a fuel cell.
“One rough estimate shows the potential inherent in this technology: At a solar performance in Germany of roughly 600 Watts per square meter, 100 square meters of this type of system is theoretically capable of storing 3 kilowatt hours of energy in the form of hydrogen in just one single hour of sunshine. This energy could then be available at night or on cloudy days.”
The scientists found out that bismuth vanadate works much better than other metal oxides in splitting water. The process of producing the solar cell is a bit complex but the materials are cheap and the results efficient, so this could be the Next Big Thing in hydrogen production (or at least one of many next big things).
Read More
http://www.helmholtz-berlin.de/pubbin/news_seite?nid=13764;sprache=en;typoid=3228
How soon before this comes online in a commercial format? It appears that none of these pieces of progress are hitting the market fast enough to warrant a serious look at the average consumer that would want them. This is a highly attractive system and takes the ‘net energy loser’ argument away from those that would shun hydrogen production that uses electricity to attain the electrolysis necessary to split h2o.
A University of Colorado Boulder team has developed a radically new technique that uses the power of sunlight to efficiently split water into its components of hydrogen and oxygen, paving the way for the broad use of hydrogen as a clean, green fuel.
The CU-Boulder team has devised a solar-thermal system in which sunlight could be concentrated by a vast array of mirrors onto a single point atop a central tower up to several hundred feet tall. The tower would gather heat generated by the mirror system to roughly 2,500 degrees Fahrenheit (1,350 Celsius), then deliver it into a reactor containing chemical compounds known as metal oxides, said CU-Boulder Professor Alan Weimer, research group leader.
As a metal oxide compound heats up, it releases oxygen atoms, changing its material composition and causing the newly formed compound to seek out new oxygen atoms, said Weimer. The team showed that the addition of steam to the system — which could be produced by boiling water in the reactor with the concentrated sunlight beamed to the tower — would cause oxygen from the water molecules to adhere to the surface of the metal oxide, freeing up hydrogen molecules for collection as hydrogen gas.
“We have designed something here that is very different from other methods and frankly something that nobody thought was possible before,” said Weimer of the chemical and biological engineering department. “Splitting water with sunlight is the Holy Grail of a sustainable hydrogen economy.”
– See more at: http://www.colorado.edu/news/releases/2013/08/01/cu-boulder-team-develops-new-water-splitting-technique-could-produce#main-content
@Dave, this looks a lot like solar molten salt reactor setup or a Heliostat setup. California has approved of a test heliostat, but nothing come of it.
Ground was broken on the project September 1, 2011.[4] Construction is expected to be complete by the summer of 2013, commercial operation planned to begin in December 2013.[5]
http://en.wikipedia.org/wiki/Crescent_Dunes_Solar_Energy_Project
http://www.solarreserve.com/what-we-do/csp-projects/crescent-dunes/
A solar performance in Germany of roughly 600 Watts per square meter, 100 square meters of this type of system is theoretically capable of storing 3 kilowatt hours of energy in the form of hydrogen in just one single hour of sunshine.
I have a few question if you can address them:
– I assume that 600 Watts isn’t W/h so what does it represent in kW/h per m2?
– A 100m2 area at 600W/m2 would translate in 60 kW (600W x 100m2). But what does that mean in kW/h?
– The 3kWh is the ability of storing part of the energy produced so one more question I have is what amount of energy is that when compared to the kW/h produced by the system?
– What’s the efficiency of this system considering the input energy and the output of the system (including the stored energy potential.
Thanks.