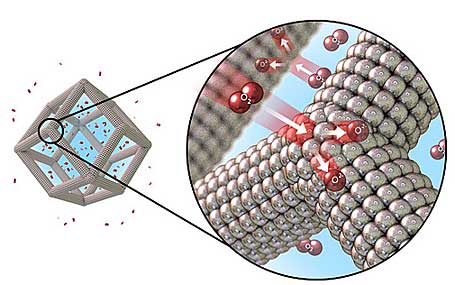
Researchers at the Argonne National Laboratory (ANL) and the Lawrence Berkeley National Laboratory (LBNL) have developed a bimetallic nanocage that can reduce the amount of platinum used in fuel cells and electrolyzers.
These 3D nanoframes made of platinum and nickel, far exceed the performance of traditional platinum-only catalysts.
According to SAE International, “The order-of-magnitude jump in performance it exhibited compared to the state-of-the-art catalysts—platinum nanoparticles deposited onto carbon substrates—is reportedly almost unprecedented. The activity of the catalyst also exceeds by an order of magnitude the 2017 target set for the technology by DOE planners.
“The bimetallic catalysts of platinum and nickel feature hollow, high-activity, high-surface-area faces both inside and out, which makes them significantly more efficient and potentially far less expensive than today’s counterparts, according to the team’s recent paper in Science magazine.
“The catalysts also work in water-alkali electrolyzers, which split water into oxygen and hydrogen and could be a potential source of hydrogen fuel depending on the cost of the electrical power to run them. Alkaline water electrolyzers have a pair of membrane-separated electrodes immersed in a liquid electrolyte made highly alkaline with caustic potash, or potassium hydroxide. The researchers tested the new electrocatalysts in the crucial cathodic hydrogen-evolution reaction, and activity was enhanced by almost one order of magnitude compared to platinum-carbon.”
On March 12, 2014 I had talked about researchers at the University of Maryland developing one-atom thick graphene nanocages that hold promise for storing hydrogen atoms. Both of the nanocage technologies that I’ve mentioned hold promise.
In fact, one day, we may be able to split water and produce hydrogen using bimetallic nanocages, store the H2 in a graphene nanocage and then release the gas into a bimetallic nanocage fuel cell to power our cars, trucks, SUVs and even off-road vehicles (which may even include a roll cage). Kind of cagey isn’t it? 🙂
Comments are closed here.